
House Lawmakers Grill FDA Over E-Cigarette Regulation and Enforcement Failures
On September 10, 2024, the House Energy & Commerce Committee held a hearing that brought forth bipartisan skepticism toward the FDA’s proposal to collect user fees from e-cigarette companies.
The FDA’s Center for Tobacco Products (CTP) aimed to gather fees during e-cigarette application submissions, similar to how traditional tobacco firms are assessed. These fees are expected to bolster the FDA’s resources, particularly in enforcing regulations against illegal vaping products.
However, lawmakers questioned the FDA’s capacity to manage these funds effectively. Concerns centered on whether the CTP, which has faced challenges in regulating the vaping industry, could deliver the necessary accountability and impact with the additional funding. Representative Larry Bucshon (R-Ind.), vice chair of the Energy & Commerce Health Subcommittee, voiced support for revisiting the issue of user fees but insisted on added accountability for how the FDA uses those funds.
The agency currently collects user fees from cigarette and cigar companies, but a congressional go-ahead is required before they can extend these to e-cigarette manufacturers. The FDA anticipates this new user fee system would inject around $114 million into their budget, with approximately half of the funds intended to ramp up enforcement efforts. Despite these proposed allocations, many lawmakers questioned how the FDA would allocate these funds in practice.
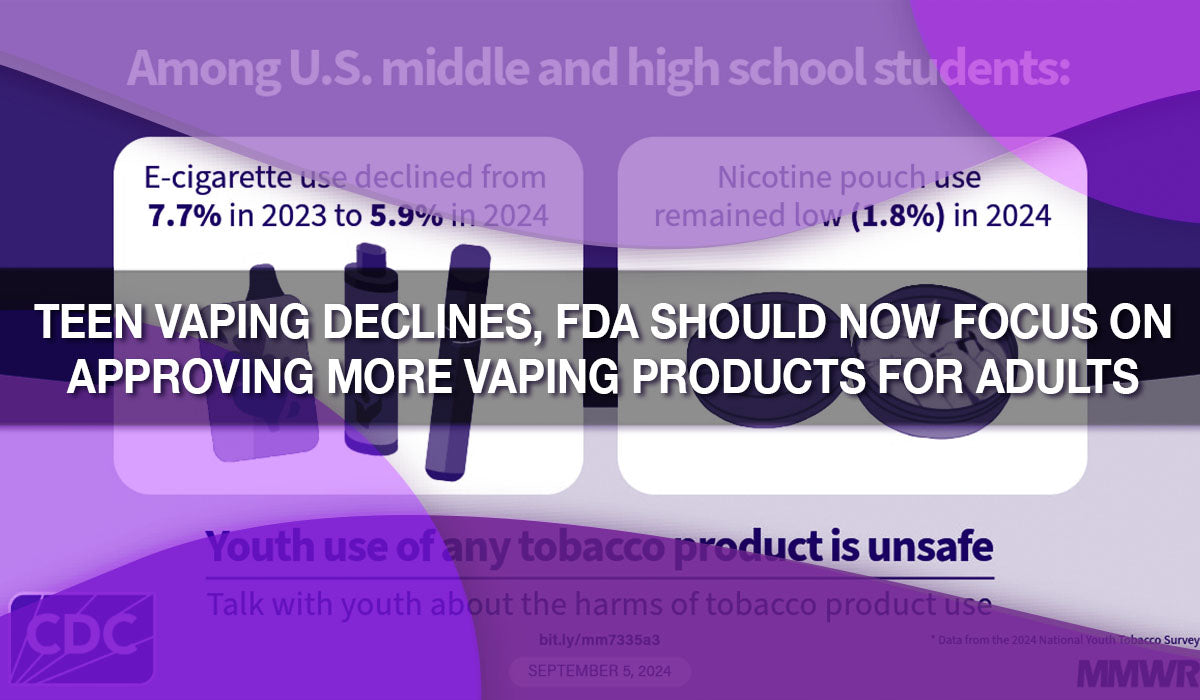
During the hearing, FDA tobacco chief Brian King defended the agency’s actions, detailing fines, seizures, and court injunctions targeting illegal vape products. But lawmakers across party lines expressed doubt over the agency’s progress in the field. Representative Michael Burgess (R-Texas) shared his frustration, pointing out the prevalence of vape shops and questioning the effectiveness of current tobacco user fees. Republicans, in particular, criticized the FDA’s conservative approach toward approving new e-cigarette products, seen as a harm reduction tool for adult smokers. Since the FDA has authorized just 34 products out of more than 26 million applications, concerns about delays and inefficiency dominated the conversation.

Lawmakers like Representative Richard Hudson (R-N.C.) suggested that the FDA should focus its enforcement efforts on larger players in the illicit vape market rather than small vape shops. He emphasized the importance of directing limited resources toward major importers and distributors for more substantial results. King, though advocating for more resources, admitted the difficulty in swiftly pulling illegal vape products off shelves nationwide.
Legislation introduced by Representative Jennifer McClellan (D-Va.) to establish the e-cigarette user fee program has yet to gain traction. With no co-sponsors and a narrowly divided Senate, the FDA’s proposal still faces hurdles on Capitol Hill. Furthermore, frustration over the FDA’s limited approvals and the persistence of illegal vape products could complicate efforts to push this initiative forward.
Rep. Larry Bucshon (R-Ind.) voiced skepticism about the FDA’s ability to use the proposed funds effectively, suggesting that the agency needs greater accountability before lawmakers consider any new fees. FDA tobacco chief Brian King defended the agency’s current efforts to police the market, citing fines against retailers and product seizures, but admitted the challenges of sweeping illegal products off store shelves quickly.
One of the most compelling moments in the hearing came during an exchange between Rep. Morgan Griffith (R-Va.) and King:
Griffith: "You’ve had 26 million applications—majority for e-cigarettes and smokeless products, which are less harmful. You’ve reviewed 99% of them, but only approved 50 products. How do you enforce this? You’re saying it’s the industry's fault that y’all haven’t gotten your work done. All but 34 have failed to provide you with the evidence?"
Griffith continued to press King:
Griffith: "We need to make it easier for you to approve these?"
King: "As a scientist, I think Congress got it right."
Griffith: "You would agree that e-cigarettes and smokeless tobacco present a lower risk than smoking?"
King: "As a general product class, yes."
Griffith: "The standard set up is keeping the product off the market. How do you protect public health if you want the safer product off the market but the less safe product on the market?"
This exchange highlighted a recurring theme throughout the hearing—the FDA’s sluggish progress in approving products scientifically found to be approximately 95% safer than smoking per a review from Public Health England or another research report published in European Addiction Research that estimated the harms of nicotine-containing consumer products and identified e-cigarettes as having about 4% of the harm of cigarettes.

As the agency continues to face mounting criticism from Congress, the future of e-cigarette regulation remains uncertain, leaving the vaping industry and its stakeholders in limbo.
With public health and industry regulation hanging in the balance, the outcome of this debate could have long-lasting implications for smokers seeking less harmful alternatives and the vape shops struggling under heavy regulations. The FDA’s next steps, particularly its ability to secure more resources and streamline the approval process, will be critical in shaping the future of the vaping industry in the United States.








Leave a comment
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.